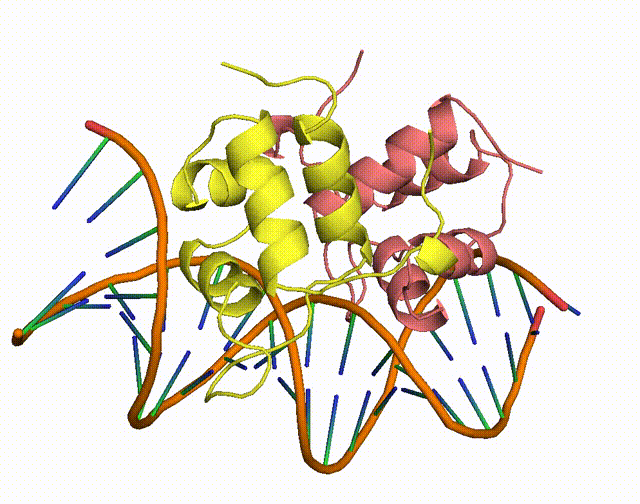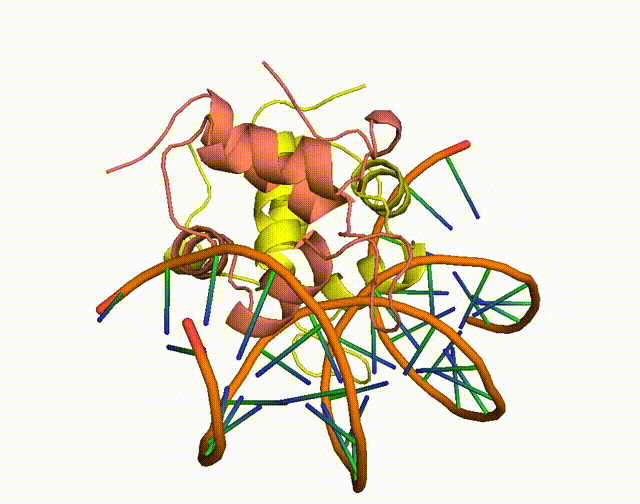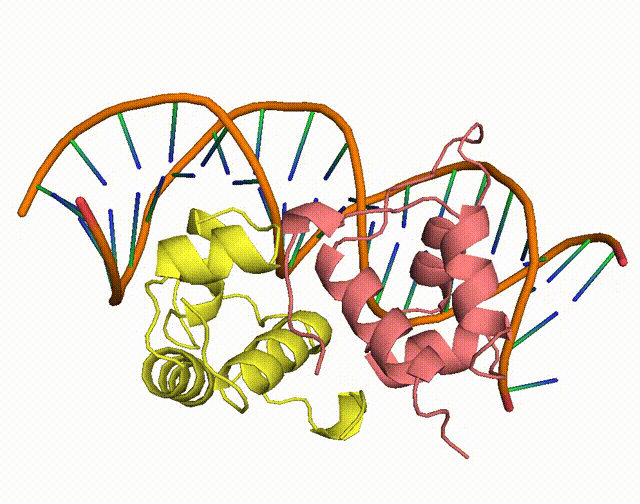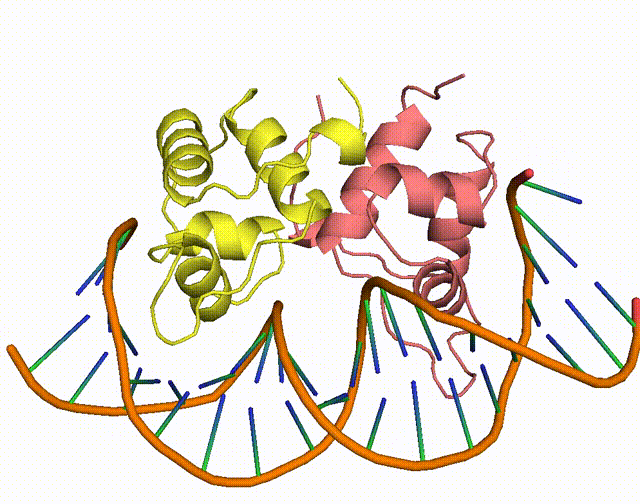Established in 1979, Shenzhen transformed dramatically from a tiny town into a modern metropolis within 41 years. Rapid urbanization brought plenty of challenges including water pollution and public health risk. To deal with this uncertain future, Shenzhen has established a program called the “Shenzhen Water Strategy”, which includes the protection of water source, the recovery of water, the guarantee of water safety, and the enhancement of visible water.
Description
Background and Inspiration
Sewage treatment has been regarded as one of the most essential parts of Shenzhen water governing strategy, and biofilm, which is composed of numerous microbes and multiple types of organic matters, plays an important role in the bioremediation of sewage. However, in this process, a common problem often arises that the pipelines of sewage treatment plants are easily blocked as a result of the overgrowth of biofilm. One proposed solution is to constantly replace the blocked pipeline. However, this method is not only too costly for large-scale applications but will also completely destroy the structure of biofilm, which can hardly be regenerated in a short period of time. Among all microbes existing in the biofilm, Pseudomonas putida contribute vastly to waste processing due to its complex metabolism. Therefore, we are inspired to design a system through which we can utilize phage infection to reduce the density of overgrown biofilm and then stop the excessive damage of phage infection using drugs that can protect bacteria from phage infection.
The conserved Aca protein in Pseudomonas phage acts on the CRISPR-Cas system in Pseudomonas. The CRISPR-Cas system is an acquired immune system in bacteria and archaea that helps them resist the invasion of foreign genetic material, such as bacteriophage, by integrating the foreign DNA sequence into its own genome1. Phages express anti-CRISPR (Acr) proteins to inhibit the CRISPR-Cas system, preventing their own genomes from being destroyed. acr genes encode proteins that bind to acr-promoter and regulate adjacent anti-CRISPR-associated (aca) genes1. Through binding to acr-promoter, Aca proteins could act as crucial inhibitors of the transcription of anti-CRISPR genes1. After the DNA of phage is injected into host cells, immediate high-level transcription of acr gene helps phage to survive under the pressure of the CRISPR-Cas system in host cells, but such high transcription level of acr gene induces dysregulation of other critical genes, leading to the death of phage1. With the presence of Aca proteins, transcription of acr gene is downregulated and phage can express essential genes for survival1. In all, if the function of Aca proteins can be artificially inhibited via small molecule compounds, we will be able to increase the defense system of the bacterium against phages.
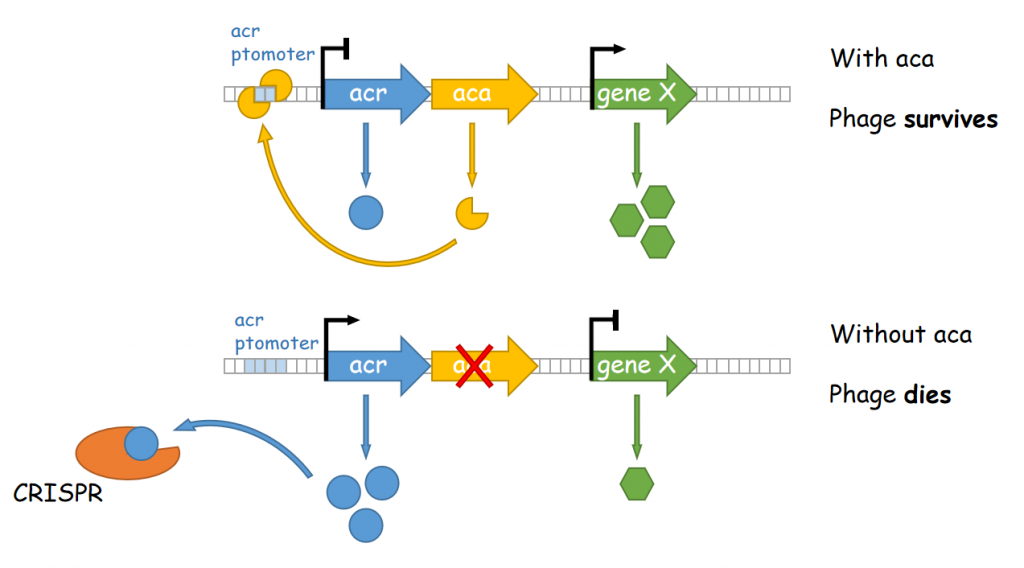
Figure 1. The working mechanism of the anti-CRISPR system
With the conserved aca genes in Pseudomonas phage, we are inspired to find an effective drug to protect Pseudomonas, an important bacterium in the sewage treatment system, from excessive phage infection in our expected system.
Project Description
As the binding between Aca1 protein and acr-promoter plays an essential role in the survival of Pseudomonas phage, we aimed to find a drug that could disrupt the binding between Aca1 protein and acr-promoter. In this way, we can combine phages that are able to reduce overgrown Pseudomonas and that drug together to regulate the state of Pseudomonas biofilm.
Based on the structure of Aca1 binding with acr-promoter, we obtained some inhibitor candidates using the Target-Directed Screening (TDS) technique. Then, we designed a two-plasmid system in E. coli to simulated the anti-CRISPR operon with the aca1 gene with egfp as a reporter. With such a system, we were able to screen potential inhibitors among those candidates. Next, we modified potential inhibitors to further improve its efficiency. Finally, we designed experiments to test the efficiency of the optimized inhibitor in protecting Pseudomonas from phage infection, but such an experiment remained unaccomplished because COVID-19 forced us to delay our laboratory work.
Reference
- Stanley, S. Y., et al. Anti-CRISPR-Associated Proteins Are Crucial Repressors of Anti-CRISPR Transcription. Cell 178, 1452-1464.e1413, doi:https://doi.org/10.1016/j.cell.2019.07.046 (2019).